Rate of Reaction Calculation
The instantaneous rate of reaction at 30 seconds 019 cm 3 s-1 is higher than the instantaneous rate of reaction at 90 seconds 008 cm 3 s-1. For example if two moles of a product were made during ten seconds the average rate of reaction would be 2 10 02 mols.
Reaction Rates And Stoichiometry Chemistry Tutorial Youtube
I Unit L-1 mol s-1 Zero order reaction.

. However this value is not technically a constant because it includes the factors that affect reaction rate. The rate of a reaction is the change in concentration of the reactant or product divided by the change in timeTherefore the formula of rate of reaction for the above reaction would be. Web As mentioned earlier the rate of reaction can be calculated a couple of different ways.
Rate of reaction 1 1 ΔA Δt 005 molL170 molL 500 s 0 s 165 molL 500 s 0003 30 molL-1s-1. K T is the rate constant or reaction rate coefficient. The rate of reaction refers to the speed at which the products are formed from the reactants in a chemical reaction.
The rate of a chemical reaction means the speed with which the reactants change into products. First the general rate of reaction formula that involves the rate constant for a general reaction equation. Or Rate K A α B β.
Values from a graph. What is the rate of reaction in science. Why is the rate of a chemical reaction important.
Web The relation between the reaction rates expressed in terms of nitrogen production and ammonia consumption for example is. If you use A to determine the rate you determine the slope of the line in the graph below. In this equation K stands for Rate constant.
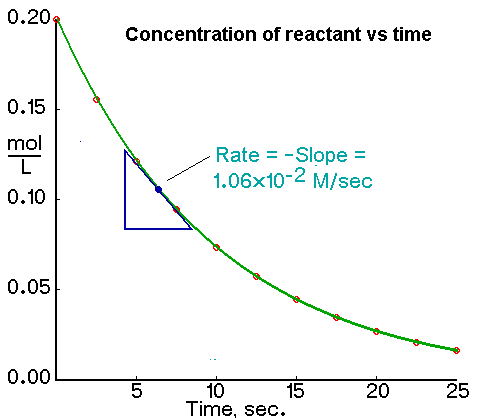
Web The average rate of reaction can be calculated using. For example the graph below could be used to calculate the average rate over any. It gives some insight into the time frame under which a reaction can be completed.
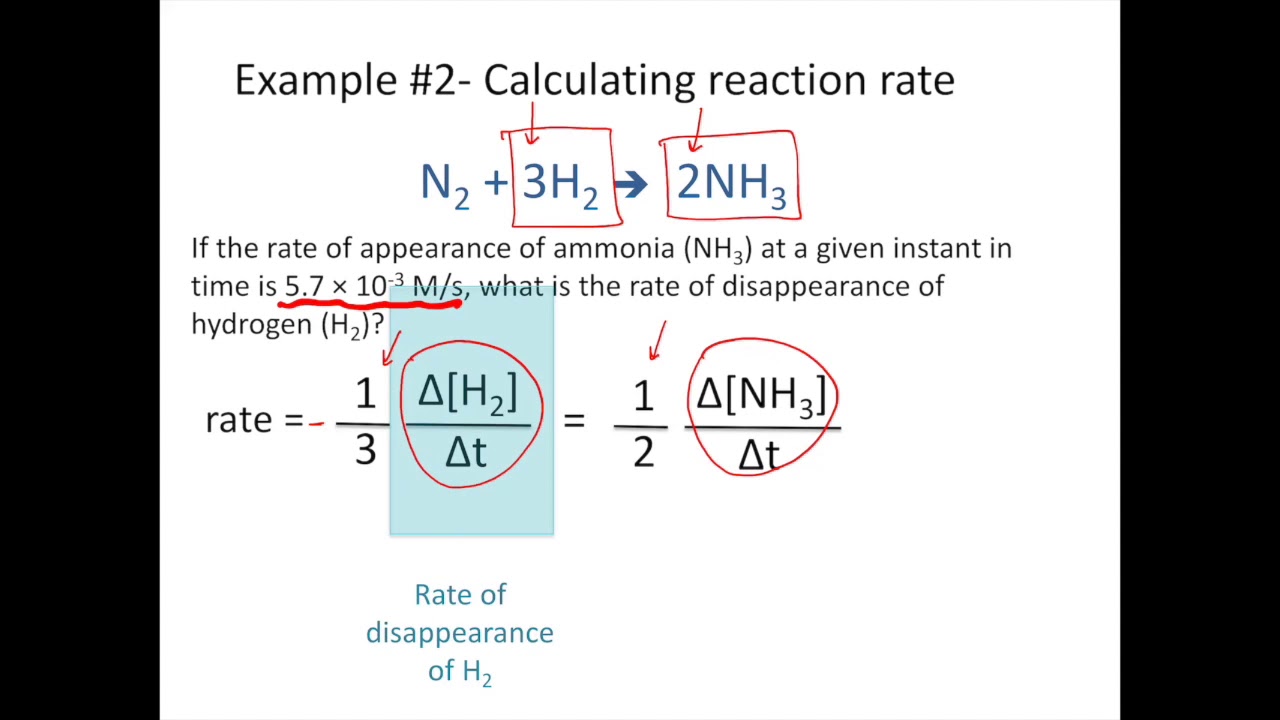
Web As the rate is changing throughout the reaction we are calculating the average rate over a given time period. Web We calculate the average rate of a reaction over a time interval by dividing the change in concentration over that time period by the time interval. Δmol NH3 Δt 1 mol N2 2 molNH3 Δmol N2 Δt Δmol NH 3 Δ t 1 mol N 2 2 mol NH 3 Δmol N 2 Δ t.
For the change in concentration of a reactant the equation where the brackets mean concentration of is. Web The rate of reaction is shown to be dependent on the concentration terms of reactant A and reactant B. Reactions have an end point.
If you use B to determine the rate you determine the slope of the line in the graph below. The steeper the line the greater the rate of. A A b B p P q Q.
Note that a negative. Ad Over 27000 video lessons and other resources youre guaranteed to find what you need. Web The rate of the chemical reaction doubles for an increase of 10 mathrmK in absolute temperature from 298 mathrmK.
Units of rate constant are. Web Factors Affecting the Rate of Reaction Rate of Reaction Formula Instantaneous Rate of Reaction. Then Rate of reaction A α B β.
The rate of reaction is the change in the concentration of any one of the reactants or products per unit time. For example the reaction rate of the combustion. Web The rate of reaction can be analysed by plotting a graph of mass or volume of product formed against time.
Web The rate of a chemical reaction can also be measured in mols. The difference in rate is due to. Web This video explains how to calculate the rate of reaction - step-by-stepIn this video we cover the following- How to measure the average rate of a reaction.
The graph shows this for two reactions. This is the end-point of the reaction meaning that. When one of the reactants has been completely used up the reaction will stop.
Web The rate of a chemical equation can be calculated using the rate equation. Web To calculate the average rate of reaction. For a chemical reaction.
To calculate the instantaneous rate of reaction. We can express this more simply without showing the stoichiometric factors units. Web We can measure the quantity of reactant used up or quantity of product formed in a reaction.
To find the average rate of reaction at 20 seconds read off the volume of product for. Data from a table of results or. We use the minus sign before the ratio in the previous equation because a rate is.
This expression is termed Rate law. R k T A n B n. The rate of the reaction is.
This is the second factor that we need to know in order to calculate the rate of a reaction. Rate of reaction - 12Δ NaΔt -Δ ClΔt 12 NaClΔt.

Calculating Rates Of Reaction 2016 Ib Biology Youtube

How Do You Calculate The Reaction Rate A Plus Topper

Introduction To Reaction Rates Video Khan Academy
0 Response to "Rate of Reaction Calculation"
Post a Comment